pharmacovigilance (PV)
Inspection / AUDIT
pharmacovigilance
Master File (PSMF) guideline
White Paper
China PV also known as Pharmacovigilance is a regulatory framework in China that prioritizes the monitoring and assurance of pharmaceutical product safety throughout their entire lifecycle. It encompasses activities such as reporting, evaluating, and managing adverse drug reactions (ADR), implementing risk management strategies, and maintaining a robust pharmacovigilance system to safeguard public health.
Scope of China PV
China Pharmacovigilance involves:
- China GVP:
Application of ICH Guidelines, Responsibilities of Regulatory Authorities, SUSAR, ICSR regulation.
- Aggregate Reporting:
This involves DSUR for clinical PV and PSUR for post-marketing PV, as well as Signal Management.
- China PV Audit and Inspection:
This encompasses the auditing and inspection procedures related to pharmacovigilance practices.
- China MAH Management:
This section addresses the management of Marketing AuthorizationHolders (MAHs), including the policy of having a local representative foroverseas MAHs.
Overlaps in China PV and EU PV
The China PV regulatory system and GVP shares similarities with the EU PV (EMA). However, there are discrepancies in requirements, including Responsible Person in Pharmacovigilance (RPPV), PV Inspection procedures, Regulatory Authorities Hierarchical Structures and others that must be addressed for successful PV Management in China.
For more on China PV, click here for our PV Guide

Pharmacovigilance in China

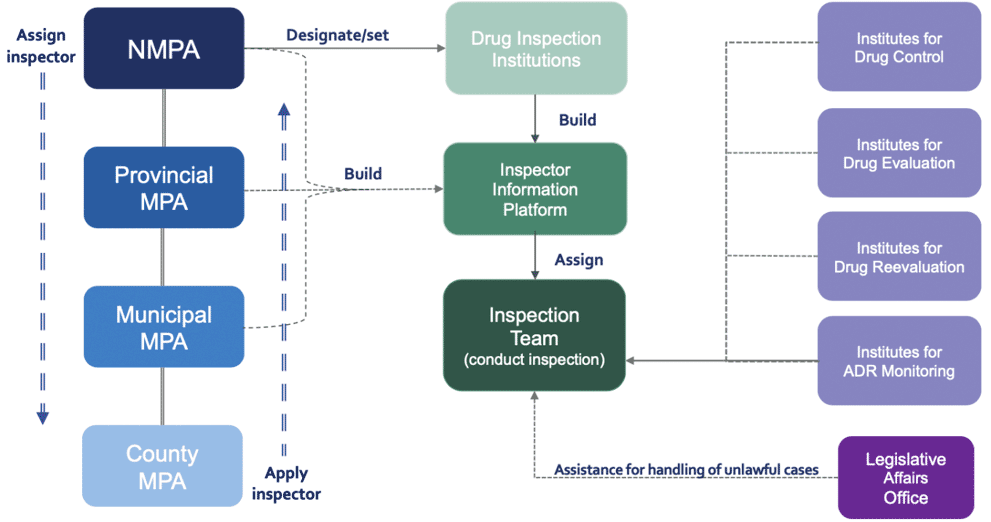
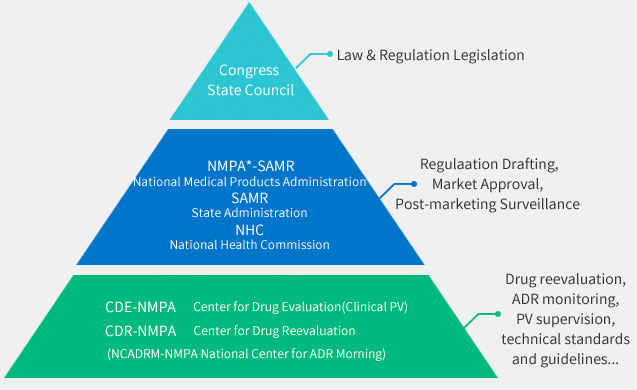
An Overview of China Pharmacovigilance Health Authorities
Guide to China Pharmacovigilance (PV) Audit
Send download link to:
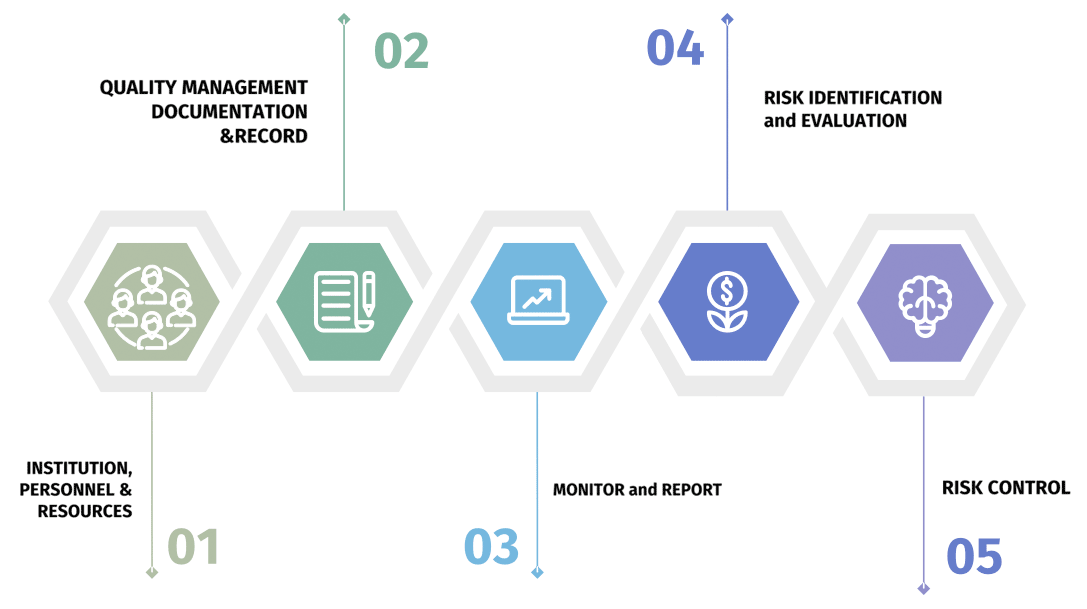
An overview component of the China's Good Pharmacovigilance Practice (GVP) similar to that of European Medicines Agency (EMA).
- Organizational Structure of MAH/Local Representative
- Responsible Person for Pharmacovigilance (RPPV)
- Specialized Personnel
- Sources of Suspected ADRs
- Information Tool/System
- Management System and SOP
- Pharmacovigilance System Operation
- Pharmacovigilance Entrustment Management
- Quality Management
- Annex
A glance at China's Regulatory Authorities that supervise and administer Pharmacovigilance related duties.
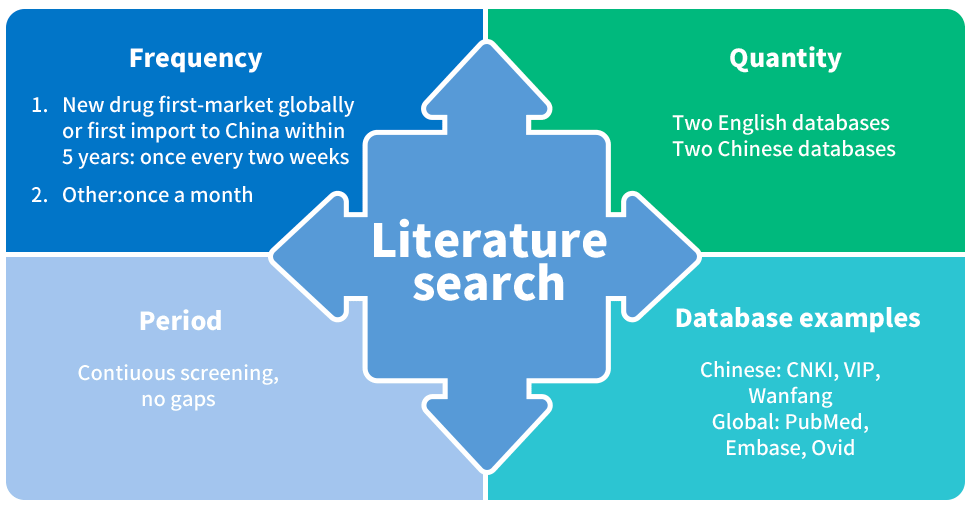
Literature Screening&lCSR in China
4 important considerations for China Literature screening for pharmacovigilance:
China ICSR process and principles:
Data Collection
Medical institutions,
manufacturers, distributors,
patients and the public,
literatures, websites/digital
media, post-marketing
safety study, etc.
Case Evaluationand Processing
1. First suspect ADR andfollow-up
2.ADR expectedness assessment
3. Causality assessment
Report
1. Timely and compliant
2. Detailed, correct andcomprehensivedescription of process ofADR and responsivemeasures
Four elements of ICSR:
1. Timeline: non-serious ADR shall be reported within 30 days and serious ADR ASAP (within 15 days) from the date of first case found by MAH.
2. ADR from literatures: submit as ICSR if suspected drug is holding by MAH; include in PSUR with analysis if not sure it is MAH’S drug.
3. Serious ADRs abroad: submit as ICSR.
4. Cases from relevant studies or data collection programs
QPPV in China is termed as RPPV which means Resnonsible Person for Pharmacoviolance the qualifications and duties are similar to EU QPPV summarized as follows:
![]() RPPV/QPPV qualifications:
RPPV/QPPV qualifications:
1)Senior management
2)Relevant education background
3)Over 3 years PV experience
4)Understand China regulations
5)Knowledge and skills for PV management
![]() RPPV/QPPV Duties:
RPPV/QPPV Duties:
1)Ensure the compliance of ADR monitor and report
2)Supervise risk identification, assessment and control, ensure theeffective implementation of risk control measures
3)Responsible for management of drug safety information communication, ensure timely and effective communication
4)Ensure smooth communication channels internally and externally(with authorities)
1.China PSMF
Since the design of China PSMF is referencing EMA PSMF template, there is plenty of similarity in terms of structures of the Master File. It is feasible to convert the required elements into the Chinese version of PSMF suitable for China Regulatory Authorities. Contact us for our PV expert for PSMF conversion consultation.
2.China PSUR
Usually globally speaking, it starts from IBD, but if no information about IBD then CBD (approval date in China). If you miss the deadline of PSUR submission, the National ADR Monitoring System will still accept the submission but it does not mean the report passes.