Drug safety is the foundation for treating patients’ diseases and a crucial factor in ensuring that patients’ lives and health are not disrupted by other factors beyond the disease. Additionally, the development of social and economic levels and the improvement of people’s health concepts, coupled with the overlapping factors of risk society and market development, have led to an increase in drug safety issues, triggering significant attention from the public and government regulatory agencies at this stage. Therefore, effective regulation of drug safety risks to achieve public drug safety has become an important issue related to the country’s well-being and the people’s livelihood. It is necessary to introduce risk control measures throughout the entire process of production, distribution, and use to safeguard drug safety.
The introduction of the drug surveillance system reflects the determination of the Chinese government to implement drug safety risk governance and a comprehensive life-cycle management concept. It provides legal guarantees for the rapid discovery and utilization of drug safety risk information. This article is based on the analysis of the background and positive significance of the drug surveillance system’s introduction in China. By discussing the existing opportunities and challenges in implementing the drug surveillance system, the aim is to explore feasible pathways to implement the drug surveillance system and provide references to ensure public drug safety.
1 Establishment of the PV System in China and the Significance of Its Implementation 1.1 Background of the Establishment of the Pharmacovigilance System
In 2019, China amended the Drug Administration Law, in which it was proposed for the first time that “the State establishes a pharmacovigilance system for monitoring, identifying, assessing and controlling adverse drug reactions (ADRs) and other harmful reactions related to the use of medicines”, highlighting the depth of the State’s implementation of the concept of risk management in the field of drug regulatory legislation, which is a major highlight of the legislative amendment. This is one of the highlights of this legislative amendment, which provides legislative guarantee for the implementation of pharmacovigilance system in China based on the current monitoring of adverse drug reactions (ADR).
In China’s previous drug safety evaluation work, academic research fields and regulatory agencies focus on the ADR monitoring work in the post-market segment of drugs. ADR information is collected, analysed and evaluated through post-market monitoring. Pharmacovigilance, as an expansion on the basis of ADR
monitoring system, focuses on issues other than post-marketing risk information monitoring of drugs, but also extends forward to the clinical trial stage of possible drug-borne damage and other issues, which is the risk control of the whole life cycle of drugs, with a strong and distinctive early warning characteristics. The construction and implementation of the pharmacovigilance system in China is based on the cornerstone of ADR monitoring, which is the objective demand and inevitable trend of the development of national drug safety evaluation.
1.2 Significance Of Establishing And Implementing A Pharmacovigilance System
The construction of a pharmacovigilance system in China is in line with the objective law of the development of drug safety monitoring. Pharmacovigilance runs through the whole process of drug research and development, approvals, market launch, use and circulation, and plays a great role in monitoring drug safety and balancing the risks and benefits of drugs. Pharmacovigilance has enriched the connotation and extension of drug safety monitoring. Compared with ADR monitoring, which emphasises the approach and process, “vigilance” focuses on the purpose and result, and its monitoring scope has also been expanded to include harmful reactions arising from drug abuse and misuse, medication errors and drug interactions, etc. In addition, compared with the single monitoring method of ADR in China in the past, pharmacovigilance is more mature and perfect in the international arena, and the commonly adopted monitoring methods include comparative observational studies, targeted clinical surveys and descriptive studies, in addition to passive and active monitoring. The establishment and implementation of the pharmacovigilance system in China is in line with the international drug safety supervision concept, and its positive significance is reflected in the following aspects.
First, it expands the regulatory calibre of drug safety risk management. The pharmacovigilance system has enriched the connotation of drug safety risk regulation, which not only includes the traditional content of ADR monitoring, but also extends to the management of human risks such as drug application, which can greatly increase the welfare of patients and show the concept of patient-centred humanism.
Secondly, it strengthens the early alert function of drug risk management. Under the adverse reaction monitoring system, due to the relative focus on post-marketing monitoring and reporting, drug risk management presents the disadvantages of “end governance”, the preventive function of the drug regulatory system is weakened, and even more unable to issue early warning information. The pharmacovigilance system promotes the “forward extension” of drug risk regulation and opens a number of links such as drug research and development, production, sales and use, providing an opportunity for the realisation of regulatory integration.
Thirdly, it promotes the participation of multiple subjects in the common governance of drug risks. Pharmacovigilance is a diversified social system comprising enterprise responsibility, government supervision, industry self-regulation, departmental coordination and public participation, and its participants are not only medical institutions and their medical staff who used to focus on ADR monitoring. The active involvement of enterprises, consumers, and other subjects in the “three-medicine linkage” of medicine, medical care and medical insurance will improve the overall effectiveness of drug risk regulation.
Table 1 A compendium of China’s pharmacovigilance legisla9on and its core content
| Time | Name | Core Content |
| 1983 | Adverse Drug Reaction Monitoring and Reporting System | Inclusion of the monitoring of adverse drug reactions as a statutory mandate for hospitals, with specific reporting responsibilities and incentives for medical staff |
| 1984 | Drug Administration Law of the People’s Republic of China | For the first time, ADR was included as an important part of drug supervision. |
| 2001 | Revision of Drug Administration Law of the People’s Republic of China | The formal establishment of the Adverse Drug Reaction (ADR) reporting system, also marked a significant increase in the degree of legalisation of Pharmacovigilance |
| 2004 | Provisions for Adverse Drug Reaction Reporting and Monitoring | China has initially established ADR monitoring systems at the national and provincial levels. |
| 2011 | Revision of Provisions for Adverse Drug Reaction Reporting and Monitoring | The responsibilities of local departments were further clarified, and a monitoring system covering the national, provincial and local (municipal) levels was established. |
| 2018 | Announcement of National Medical Products Administration (NMPA) on Direct Reporting of Adverse Drug Reactions by Drug Marketing Authorization Holder (MAH) (No. [2018] 66) | MAH are required to establish a sound monitoring system for adverse drug reactions and to report all adverse reactions they are aware of in a timely manner. |
| 2019 | Revision of Drug Administration Law of the People’s Republic of China | China established a pharmacovigilance system to monitor, identify, assess, and control adverse drug reactions and other harmful reactions related to the use of medicines. |
| 2020 | NMPA Announcement on the application of the ICH Guidelines, E2C(R2): PBRER (No. [2020]86) | Promoting technical standards for drug regulation in line with international standards, providing support for strengthening the management of the whole life cycle of drugs and optimising the reporting of periodic safety update reports by marketing authorisation holders. |
| 2020 | Revision of Provisions for Drug Registration | Sponsors are required to report to the Centre for Drug Evaluation (CDE), NMPA regarding information on Suspected and Unexpected Serious Adverse Reactions (SUSAR) and other potentially serious safety risks during clinical trials of drugs. |
| 2021 | China Good Pharmacovigilance Practice (GVP) | Establishes the primary responsibility of MAH and clinical trial applicants for pharmacovigilance actions and regulates and guides them in the conduct of pharmacovigilance activities. |
| 2022 | Guidelines for Pharmacovigilance Inspections | Provides guidance to regulatory authorities on the conduct of pharmacovigilance inspections. |
2 Opportunities and Challenges of Implementing a Pharmacovigilance System in China
2. 1 Opportunities for Implementing Pharmacovigilance Systems
2. 1. 1 Fuelled by National Policy and Social Philosophy
As the leading force in drug safety regulation, the government is responsible for controlling drug risks, and its goal is to achieve well regulation in drug safety management. From the 1980s, when China began to devote its focus to ADR monitoring, to the current national pharmacovigilance system established in accordance with the law, the relevant legislation and policies have been revised and introduced several times, with the aim of strengthening drug safety monitoring in keeping with the times and in line with the concept of putting people’s lives and health at the core.
The implementation of the pharmacovigilance system in China has possessed very favourable conditions, including, but not limited to, clear and detailed vigilance responsibilities of MAH and clinical trial applicants, as well as clear regulatory procedures of the regulatory authorities, which have provided compliance support for the monitoring of risk signals, regular safety update reports, post-marketing safety studies and other actions to make up for pharmacovigilance. In addition, China’s accession to ICH and the gradual transformation and implementation of its relevant technical standards and guidelines reflect the continuous advancement of drug safety regulatory concepts to the international arena, and the application of ICH guidelines provides experience and guidance for the development of vigilance activities in China. The legislation on pharmacovigilance in China is summarised in Table 1.
The collection and analysis of suspected ADR information is an important part of the work under the pharmacovigilance framework, and the principle of “report on suspicion” has enabled the existing ADR
Monitoring Centre database in China to collect ADRs caused by factors such as the use of medicines, which exceeds the definition of the scope of ADR information in the statutory context, and provides data support for the monitoring of suspected ADR information in pharmacovigilance practice. ADR information monitoring provides data support. In 1998, China joined the World Health Organization (WHO) Uppsala Monitoring Center (UMC), and in the following year, China set up the National Adverse Drug Reaction Monitoring Centre (NADRMC), which is responsible for the monitoring of ADRs. In the following year, China set up the National Adverse Drug Reaction Monitoring Centre (NADRMC), which undertakes the monitoring of ADR and the formulation of technical standards and norms, as well as the provision of technical guidance to local monitoring and post-marketing safety evaluation. A four-tier ADR monitoring system covering the national, provincial, municipal and county levels has been set up to carry out professional and technical analyses and evaluations of the collected ADR information, providing solid technical support for the smooth implementation of post-marketing drug safety monitoring in China.
In addition, at the level of ADR reporting, the total number of reports in China has been increasing over year, gradually shifting the format of reporting from paper to electronic and expanding the main body of reporting from the pilot hospitals to the places of drug production, operation and use in the country. In 2021, the average number of ADR reports in China is 1,392 per million population, which is close to the level of developed countries, and is an important proof of the improvement of the level of monitoring work. The data of the UMC report for 2021-2022 shows that among the ICSRs received by its ADR database (VigiBase), the number of China’s reports accounted for 4 per cent of the world’s total number of reports1 (Figure 1).
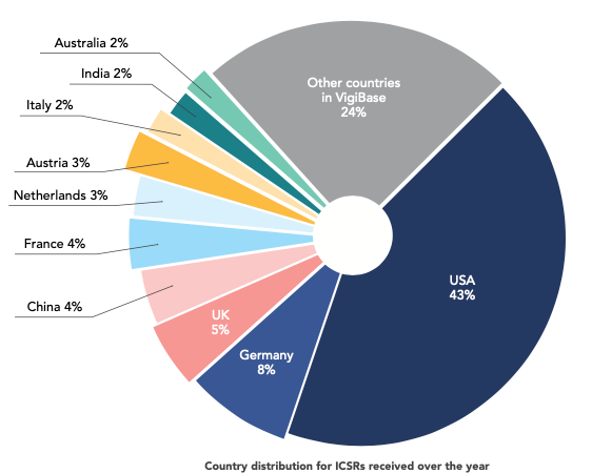
Figure 1. Country distribution in VigiBase for ICSR received during the past 12 months, as of 30-06-2022.
Reference
1. Uppsala Monitoring Center. UMC Annual Report 2021-22 https://who-umc.org/media/cgnlrs5v/umc-annual-report-2021-22.pdf
Contact Us
For more informa[on or request for solu[ons that tailored to your product, please email: info@accestra.com
