Comparison Between China PV and Europe PV
Comparison of China GVP and EMA GVP

The European Medicines Agency (EMA) has developed 12 modules (I to XVI)
for pharmacovigilance processes. The EMA has already covered the topics
for Modules XI to XIV in other guidance documents, and thus they are null.
The remaining modules play a vital role in ensuring patient safety.
Module I focuses on quality objectives for pharmacovigilance systems to
comply with safety standards. It guides pharmaceutical companies on how to
handle each aspect of the process to meet those objectives.
Pharmacovigilance systems are used to identify if a drug has adverse
effects and to track drug safety over time. Organizations can show their
findings to regulatory authorities and alert the public if new adverse
events are detected and verified.
Module II requires the inclusion of a pharmacovigilance system master file
(PSMF) with every marketing authorization (MA) application. The PSMF must
contain details on the Qualified person responsible for pharmacovigilance,
organizational structure, computer system, PV processes, quality system
activities, delegated activities.
Module III and IV are pharmacovigilance inspections and audits
respectively, which are conducted by competent authorities to ensure
compliance with EMA guidelines. Inspections ensure the availability of
resources to meet pharmacovigilance requirements, while audits verify the
ability of pharmacovigilance systems to perform activities effectively.
Module V involves the creation of a risk management plan (RMP) for every
drug. The RMP identifies the safety profile of the drug and creates a
pharmacovigilance plan to identify new adverse reactions. As more
information emerges, the RMP adapts to changes in the drug’s safety
profile.
Module VI provides guidance on the collection, management, and submission
of reports of suspected adverse reactions to medicinal products. The
reports are collected by pharmacovigilance systems and evaluated by
competent authorities and MA holders.
Module VII focuses on Periodic Safety Update Reports (PSURs) which provide
an overview of a drug’s risk-benefit during the post-authorization phase.
The competent authorities review the PSURs within 70-90 days of the data
lock point to check if the risk-benefit balance has changed.
Module VIII involves conducting Post-Authorization Safety Studies (PASS)
to identify a safety hazard or confirm the safety profile of a drug. These
studies are initiated by the EMA or the MA holder and reviewed by the
Pharmacovigilance Risk Assessment Committee (PRAC).
Module IX refers to signal management which is about the evaluation,
reporting, and timelines of drug safety issues. Pharmaceutical companies
need to send a signal to the EMA within 30 days of identifying an adverse
reaction.
Module X involves additional monitoring for certain drugs that may have
adverse reactions emerge after authorization. The EMA maintains a list of
these drugs which require additional data collection, and they are
identified with an inverted equilateral black triangle ▼.
Module XV is about safety communication, and it involves disseminating
information to healthcare professionals and the public about the safe use
of drugs.
Module XVI focuses on the implementation of risk minimization measures to
minimize risks associated with drug use. These measures are based on the
RMP and are regularly reviewed and updated. Overall, these modules help in
ensuring the safety of patients and the public.
China GVP on the other hand showed similar structures. China GVP is based
on the “Guidelines for Adverse Drug Reaction Reporting and Monitoring”
issued by the Chinese Ministry of Health, while EU GVP is developed by the
European Medicines Agency (EMA) in collaboration with the EU member
states.
In terms of content, both frameworks cover similar aspects as illustrated
in the graph with same colour highlighted for the PV aspects covered.
However, there may be differences in specific requirements and guidelines
due to variations in regulatory processes and regional considerations. It
is important for pharmaceutical companies operating in both regions to be
familiar with and adhere to the respective GVP guidelines to ensure
compliance with local pharmacovigilance requirements.
Should you have any queries or seeking for consultation, please contact
us via online chat or email.
China PSMF vs EMA PSMF
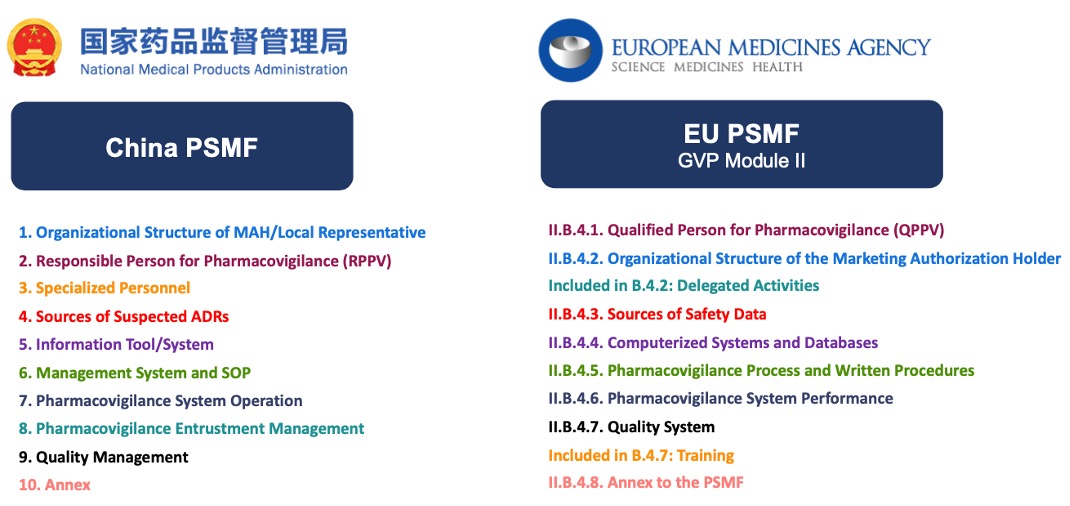
The establishment of China PSMF referenced EMA GVP Module II of PSMF as a
model. Nevertheless, two additional aspects “Entrustment of
Pharmacovigilance Activities” and “Full-time Personnel” are incorporated
in the China PSMF as required in the Guideline for Preparation of the
Pharmacovigilance System Master File published by NMPA.
Should you have any queries or seeking for consultation, please contact
us via online chat or email.
Responsible Person for Pharmacovigilance – A Thorough Analysis of Current
China “QPPV” System
The implementation of Pharmacovigilance activities in China is governed by
the China (GVP), which mandates Marketing Authorization Holders (MAHs) to
establish pharmacovigilance quality objectives and a comprehensive quality
assurance system. To fulfill PV responsibilities, MAHs are required to
introduce the concept of the Qualified Person for Pharmacovigilance
(QPPV). Similarly, the European Union requires MAHs to establish a
Pharmacovigilance system and appoint a QPPV. However, the United States
does not have explicit requirements for Pharmacovigilance systems.
The official implementation of the China GVP is on December 1, 2021,
emphasising the primary responsibilities of MAHs in Pharmacovigilance.
Despite the existence of Level 3 Adverse Drug Reaction Monitoring Centres,
a shortage of QPPVs has been observed in recent years in China.
The qualifications and responsibilities of QPPVs within the GVP quality
management system have led to variations and confusion within MAHs. This
includes differences in position designation, hierarchical level, duties,
and the required knowledge and skills for QPPVs. The QPPV plays a vital
role as the main contact between the MAH and Regulatory Authorities,
ensuring drug safety and compliance with regulations through the
establishment and maintenance of pharmacovigilance systems, monitoring and
reporting adverse drug reactions, and implementing risk management
strategies.
Analysis of QPPV Position Requirements in China – General Requirements for
QPPV Qualifications
In China GVP it is required that the QPPV “should be a managerial position
with certain responsibilities, have a background in medicine, pharmacy,
epidemiology, or related fields, possess a bachelor’s degree or higher
education, or have an intermediate or higher professional and technical
title.” It also states that the QPPV should have “over three years of
experience in pharmacovigilance-related work, be familiar with
Pharmacovigilance-related laws and regulations and technical guidance
principles in China, and have the knowledge and skills for
Pharmacovigilance management”.
The selection requirements of EU QPPV also include similar professional
background requirements, with a specific emphasis on medical knowledge or
a background in medicine. The EU-GVP mentions that the EU-QPPV should
possess the skills to manage the pharmacovigilance system and have the
expertise or ability to acquire knowledge in relevant fields such as
medicine, pharmacy, epidemiology, and biostatistics.
Analysis of QPPV Position Requirements in China – Professional and
Technical Responsibilities of QPPV
In China GVP, it is required that the QPPV should have “over three years
of experience in pharmacovigilance-related work,” meaning that they have
been dedicated to working in drug vigilance and have experience in
activities related to the collection and reporting of drug safety
information (such as physicians, pharmacists, CRA/CRC personnel in
clinical research), as well as adverse drug reaction (ADR) handling.
Similarly, in the EU-GVP, EU-QPPV applicants or MAHs are subject to
qualification review and assessment before appointment, including
evaluation of university education, understanding of EU pharmacovigilance
requirements, and experience in pharmacovigilance.
According to Article 25 of the China GVP, the QPPV is responsible for the
operation and continuous improvement of the pharmacovigilance system,
ensuring its compliance with relevant laws, regulations, and the
requirements of the GVP. Therefore, the responsibilities of the QPPV can
be summarized as continuous improvement, compliance, risk control,
information communication, and auditing.
- The foundation of pharmacovigilance quality management work is based
on the pharmacovigilance system as the main focus. The QPPV implements
continuous improvement plans through the Deming cycle (Plan, Do,
Check, Act), ensuring the effective operation of the pharmacovigilance
system.
- Compliance of monitoring reports is one of the quality objectives of
pharmacovigilance. By setting quantifiable quality control indicators,
the effectiveness of the pharmacovigilance system is measured. If the
compliance indicators are not met, the QPPV should lead the analysis
of errors, training, processes, and other reasons.
- Pharmacovigilance is work based on the principle of “full-process
control and risk management.” The QPPV should supervise the entire
process of pharmacovigilance, conduct drug safety risk identification,
assessment, and control, and ultimately ensure the effective
implementation of risk control measures.
- Channels of communication in pharmacovigilance should remain open,
including multiple channels for reporting safety information (phone,
fax, email, mobile applications, etc.). QPPV should be contacted
(department) promptly through convenient means to provide feedback on
safety issues or other work instructions, such as conducting
inspections and benefit-risk assessments, and respond adequately and
timely to inquiries from Regulatory Authorities.
- Safety information is one of the important contents of
pharmacovigilance work. The QPPV is responsible for the accurate and
complete communication and transmission of information to ensure
timely and effective communication.
- The QPPV needs to review and issue important documents, including
periodic safety update reports and post-marketing safety study
protocols. These documents reflect the integrity of the
pharmacovigilance system or the safety of specific products. However,
it is not recommended to include Individual Case Safety Report files
in the category of important documents.
Comparison of QPPV Positions between China and the European Union –
Qualifications for Appointment
There are some differences in the qualifications for QPPV positions
between China and EU, mainly in terms of experience, knowledge and skills,
and personnel attributes (see Table 1).
- Education and professional background: No significant differences
between China and EU. Both require a Bachelor degree or above, with a
focus on relevant fields such as medicine and pharmacy. However, the
EU places more emphasis on the field of biostatistics. Due to the
interdisciplinary nature of Pharmacovigilance, both China and the EU
face a shortage of professionals in the field, allowing individuals
with relevant backgrounds to assume QPPV responsibilities.
- Experience and qualifications of QPPV: China specifies a requirement
of 3 years of relevant experience in pharmacovigilance, while the EU
does not explicitly mention the years of experience.
- Knowledge and skills: Both China and the EU require familiarity with
local pharmacovigilance-related laws, regulations, and technical
specifications. In China, as the initial version of GVP was just
released in May 2021, it may take some time for QPPVs to acquire the
necessary knowledge in the field of drug vigilance. Therefore, if
Chinese pharmaceutical companies choose to hire or outsource QPPV
positions, it may be possible to quickly meet legaland policy
requirements.
- Duties and place of residence: China requires QPPVs to hold management
positions and expects them to influence relevant personnel and
departments through these positions, which is more conducive to
conducting Pharmacovigilance work. The EU-GVP does not specify
management position requirements, although it emphasizes
responsibilities and influence, which typically requires time
accumulation in managerial roles. The EU also emphasizes that QPPVs
should reside within the EU or in Norway, Iceland, or Liechtenstein.
China does not mention residence requirements, but in general, it is
preferable for QPPVs to be located within China, as it facilitates
communication with Regulatory Authorities.
- Personnel attributes of QPPV: The Chinese GVP does not mention the
outsourcing or employment of external QPPV personnel, while the EU-GVP
allows QPPVs to be employees of third-party companies to oversee and
manage the pharmacovigilance system of MAHs.
QualificationChina RPPVEU-QPPV
| Educational Backgraound |
Bachelor or Mid-level and above Technical Titles |
University/College |
| Major |
Medicine, Pharmacy, Epidemiology or related majors |
Medicine, Pharmacy, Epidemiology, Biostatistics and others |
| Knowledge/Skill |
Familiar with China’s pharmacovigilance-related laws and regulations
and technical guidelines, with knowledge and skills in
pharmacovigilance management |
Familiarity with EU pharmacovigilance requirements |
| Experience |
At least three years of experience in pharmacovigilance related work |
Experience in pharmacovigilance required |
| Roles |
Managerial positions |
May be an independent individual, not mentioned as requiring a
managerial position |
| Residence |
not mentioned, generally within China |
Norway, Iceland, or Liechtenstein |
| Personnel Attibute |
Not mentioned if non-MAH employee is allowed |
MAH Employee or Entrusted |
Table 1 Comparison of QPPV Qualification between China and EU.
Comparison of QPPV Positions between China and the European Union – Job
Responsibilities
There are differences in job responsibilities between QPPV positions in
China and the European Union, including compliance management,
communication management, government-enterprise communication, as well as
management and legal responsibilities. The initial version of GVP in China
provides a relatively concise and clear description of QPPV
responsibilities. In Article 25, under “Personnel and Training,” it
briefly outlines the responsibilities of QPPV, such as
compliance,supervision, communication management, government-enterprise
communication, and review and issuance. The Pharmacovigilance System
Master File (PSMF) also requires basic information about the QPPV,
including residence area, contact information, resume, and
responsibilities. On the other hand, the European Union’s GVP provides a
more detailed introduction to the responsibilities and role of the QPPV.
The supervisory responsibilities include standard operating procedures,
contract arrangements, database operations, compliance data related to
quality, regular updates and auditing reports, completeness and timeliness
of expedited reporting, training on pharmacovigilance, and contracted
management. Please refer to Table 2 for more details.
Regarding the management responsibilities of the PSMF, the Chinese GVP
does not mention or emphasize the QPPV’s authority over PSMF management or
specify the personnel responsible for reviewing and issuing the PSMF.
However, as a system description document, the European Union particularly
emphasizes the QPPV’s management authority over the PSMF. In terms of
legal responsibilities, there are differences between China and the
European Union in terms of specific legal obligations. In China, the QPPV
is primarily responsible for technical guidance, specifically for the
technical aspects of drug vigilance,without mentioning the need to assume
corresponding legal responsibilities and risks. On the other hand, the
European Union QPPV has legal responsibilities, meaning that the position
carries certain risks, and it is necessary to ensure compliance with legal
requirements to avoid personal and company penalties. Therefore, based on
the practical experience of QPPVs in the European Union in terms of
quality improvement, management responsibilities, and legal regulations,
as well as the early stage of establishing the drug vigilance system in
China, it is necessary for China to develop more detailed specifications
for QPPV job responsibilities. These specifications can be used by
pharmaceutical companies and MAHs to implement QPPV selection from the
perspectives of risk management and compliance management.
Outsourcing Analysis of QPPV – Current Human Resources Situation of QPPV
in China
Currently, there are no specific laws and guidelines in China that
explicitly state whether MAHs can outsource QPPV or whether QPPV should
assume legal responsibilities. Possible reasons for this could be based on
the experience of implementing Good Manufacturing Practices (GMP) and Good
Supply Practices (GSP), where key personnel responsible for quality
management should be full-time employees of the company, without
precedents for outsourcing or hiring third-party personnel. At the same
time, considering the current status of practicing pharmacists and Risk
Management of MAH in China, no sufficient QPPVs who truly meet regulatory
requirements to meet market demand. It may also be considered that most
companies can initially meet transitional standards, and regulations and
norms only explicitly state that the entity’s responsibility lies with the
legal entity or the primary person in charge, without requiring the QPPV
to assume legal responsibilities.
When implementing outsourcing for a company’s pharmacovigilance program,
it is possible to consider engaging third-party personnel to assume the
responsibilities of QPPV on behalf of the MAH. In this case, there is no
labor contract between the MAH and the QPPV, but rather a service contract
that binds them. This method of outsourcing QPPV hiring can draw lessons
from the QPPV system in the European Union.
Outsourcing Analysis of QPPV – Relevant Regulations on Pharmacovigilance
Agreements
With the advancement of GVP learning and exchange both domestically and
internationally, as well as the gradual maturity of MAH outsourcing
business, QPPV entrusting may become an important component of MAH’s
quality system outsourcing. In the second chapter of China’s GVP, titled
“Entrustment Management,” MAH is required to “entrust
pharmacovigilance-related work based on job needs,” but responsibilities
are not transferred, and “corresponding legal responsibilities are borne
by the holder.” In 2020, the National Medical Products Administration
issued the “Guidelines for Writing Pharmacovigilance Agreements (Trial),”
but the document does not mention entrustment related to QPPV.
Reference:
1. Discussion and Thoughts on the Job Responsibilities and Selection of
QPPV between China and EU, XU Juping, HU Jun, WAN Bangxi, WEI Xiaofei,
WANG Guangping
Should you have any queries or seeking for consultation, please contact
us via online chat or email.
Individual Safety Case Report
NMPA
Reporting timelines:
- Fatal ADRs: immediately (For cases received during the weekend, submit
within next business day, no later than Day 3)
- Other serious ADRs: within 15 calendar days
- Non-serious ADRs: within 30 calendar days.
EMA
Submit the valid ICSR (EEA and non-EEA serious within 15 days, and EEA
non-serious ICSR within 90 days to EudraVigilance (EV)). Non-serious
non-EEA ICSRs should not be submitted to EV.
FDA
Must report adverse drug experience that is both serious and unexpected,
whether foreign or domestic, as soon as possible but no later than 15
calendar days from initial receipt of the information and must submit
follow-up reports within 15 calendar days of receipt of new information by
the applicant.
The applicant must report each adverse drug experience involving serious
listed, non-serious unlisted and listed events at quarterly intervals, for
3 years from the date of approval of the application, and then at annual
intervals. The applicant must submit each quarterly report within 30 days
of the close ofthe quarter (the first quarter beginning on the date of
approval of the application) and each annual report within 60 days of the
anniversary date of approval of the application. Follow-up information to
adverse drug experiences submitted in a periodic report may be submitted
in the next periodic report.

