ICH and PV
Understanding the Role of ICH in Drug Safety and Pharmacovigilance
The International Council for Harmonisation (ICH) was established in 1990 as a
collaboration between regulatory authorities and industry professionals to
ensure the safety, quality, and efficacy of medicines intended for human use.
The need for greater uniformity in testing and safety regulations across
regions led to the formation of ICH, which has been able to develop practice
guidelines that meet global standards through working groups comprised of
representatives from regulatory authorities, pharmaceutical industries, and
organizations such as the World Health Organization.
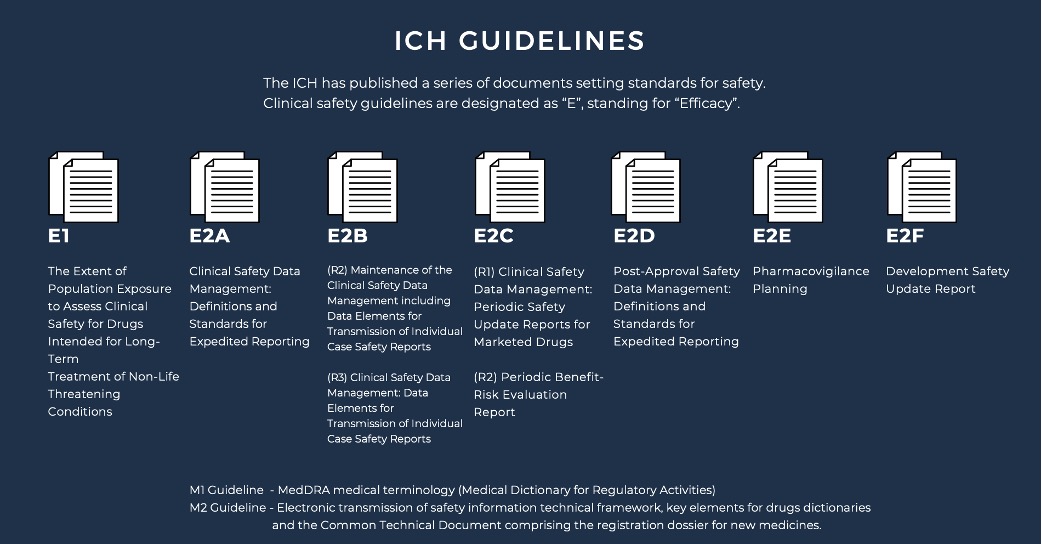
The ICH has established numerous documents outlining safety standards for both
pre-clinical and clinical stages of drug development. Pre-clinical guidelines
are identified with an “S” designation, while clinical safety guidelines are
marked as “E” for “Efficacy.” Each clinical safety guideline has a unique
identifying code, which is periodically revised to reflect updated standards.
The ICH has developed several clinical safety guidelines that have reached
“step 4” status and provide detailed expectations for reporting clinical data
to regulatory authorities.
E2A Clinical Safety Data Management provides definitions and
standards for the expedited reporting of adverse drug reactions (ADRs) to
regulatory authorities to ensure patient safety. E2B (R2) Maintenance of the
Clinical Safety Data Management including Data Elements for Transmission of
Individual Case Safety Reports provides a standardized format for the
maintenance and transmission of individual case safety reports (ICSRs) to
regulatory authorities to ensure timely and accurate reporting of ADRs.
E2B (R3) Clinical Safety Data Management: Data Elements for
Transmission of Individual Case Safety Reports updates and expands on the
previous E2B (R2) guideline, providing a more comprehensive list of data
elements to be included in ICSRs.
E2C (R1) Clinical Safety Data Management: Periodic Safety
Update Reports for Marketed Drugs provides guidance on the content, format,
and frequency of periodic safety update reports (PSURs) for marketed drugs,
with the aim of ensuring ongoing assessment of drug safety.
E2C (R2) Periodic Benefit-Risk Evaluation Report provides
guidance on the preparation and submission of periodic benefit-risk evaluation
reports (PBRERs) for marketed drugs, with the aim of ensuring ongoing
assessment of the balance between drug efficacy and safety.
E2D Post-Approval Safety Data Management: Definitions and
Standards for Expedited Reporting provides definitions and standards for the
expedited reporting of ADRs that are identified post-approval, with the aim of
ensuring ongoing monitoring and assessment of drug safety.
E2E Pharmacovigilance Planning provides guidance on the
development of pharmacovigilance plans for new drugs, with the aim of ensuring
proactive monitoring and assessment of drug safety.
E2F Development Safety Update Report provides guidance on the
preparation and submission of development safety update reports (DSURs) for
drugs in clinical development, with the aim of ensuring ongoing assessment of
drug safety during the clinical trial process. Please note that this page
cannot provide detail on the full scope of the ICH guidelines and the
interested reader is referred instead to the source material which can be
found online at the ICH website, detailed in the references below. Please note
that this page should not be considered as professional pharmacovigilance
advice.
Application of ICH in China Pharmacovigilance
According to CFDA Announcement on the Application of Secondary Guidelines of
International Conference on Harmonization Technical Requirement for
Registration of Pharmaceuticals for Human Use (No.10, 2018)
- From 1 May 2018, E2A, M1, E2B(R3) applied for SUSAR in Clinical studies of
medicinal products
- From 1 July 2018, E2D applied for the reporting of post-marketing ADR
- From 1 July 2019, the requirement of M1 and E2B (R3) can be applied for
post-marketing ADR and from 1 July 2022, above technical guidelines (M1
and E2BB (R3)) applied to the reporting of post-marketing ADR.
According to Announcement by NMPA on the Application of 15 ICH Guidelines,
including “E1: Exposure Levels in Populations: Assessing the Clinical Safety
of Drugs for the Long-Term Treatment of Non-Life-Threatening Diseases” and
others (No. [2019] 88)
- E2F will be applicable from the Announcement date (12 Nov 2019)
- E2E: will be applicable to NDA accepted 3 months after the Announcement
date & NDA approved 6 months after the Announcement date